About Ensho Therapeutics
Ensho means inflammation, flame, or glow in Japanese and expresses our deep desire to deliver breakthrough oral therapies to patients with inflammatory diseases. We believe our pipeline of oral, small molecule inhibitors of the integrin α4β7 could be transformational for patients suffering from inflammatory bowel disease (IBD).
In the first half of 2025, we plan to initiate a Phase 2 clinical program for our lead drug candidate, NSHO-101 (also known as EA1080). We believe we can provide an oral therapeutic solution for α4β7 inhibition, a mechanism that has already been shown to be safe and effective by a commercially available antibody, but where no oral options currently exist. For the millions of patients who suffer from IBD, we hope to extinguish their inflammation and provide relief from this difficult-to-treat disease.
Quiet the Fire Within
Millions of people in the Unites States are living with IBD, and while there are a number of approved medications to address the symptoms of IBD, it remains a difficult-to-treat disease with high relapse rates.
The chronic inflammation that is a hallmark of IBD ultimately damages the gastrointestinal tract. We believe that an oral agent that works selectively in the gut could inhibit damage, providing a safe and effective therapeutic option to the many patients who are not adequately treated today.

Our Pipeline
Our pipeline of oral, selective, small molecule inhibitors of the integrin α4β7 provides multiple opportunities to address a number of gastrointestinal diseases, including ulcerative colitis (UC) and Crohn’s disease.
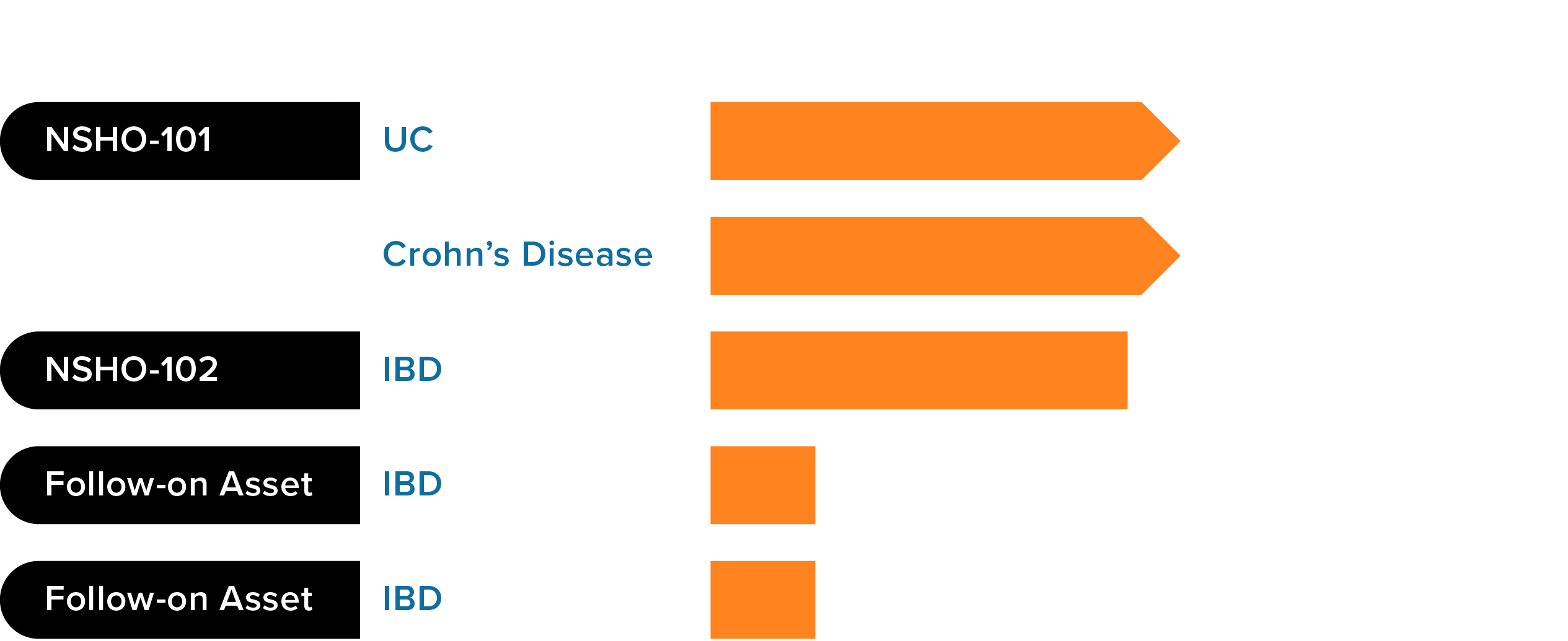
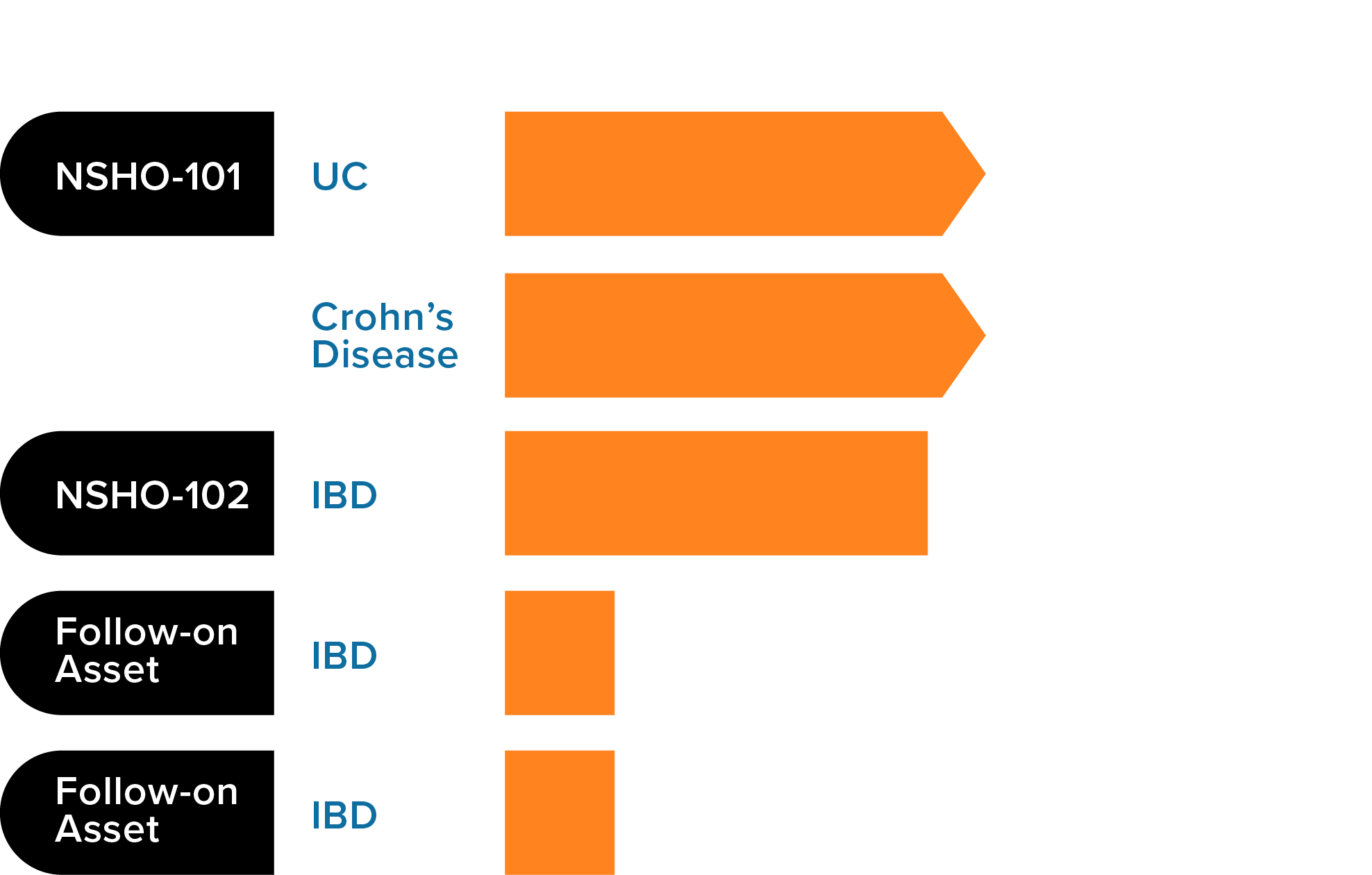
Our drug development candidates are oral agents designed to inhibit α4β7 activity and quell inflammation
α4β7 is a cell surface receptor that helps regulate the migration of immune cells to the intestine and plays a key role in controlling inflammatory responses. It binds to mucosal addressin cell adhesion molecule-1 (MAdCAM-1), which is expressed on intestinal venules and is upregulated in response to inflammation. This interaction facilitates the transport of leukocytes and recruitment of effector lymphocytes to the gut mucosa in IBD. Because α4β7 is found primarily in the gut, this localized action provides immune cell inhibition where it is most relevant, potentially minimizing side effects and preserving overall immune cell function.
Decades of research validate α4β7 inhibition as an anti-inflammatiory mechanism in IBD, which has been further substantiated by the approval of a commercially available antibody, vedolizumab. Yet, there are no approved orally administered α4β7 inhibitors. That’s where our pipeline of oral, selective, small molecule inhibitors of α4β7 can play a role.
Our lead drug candidate
NSHO-101 is a novel, oral, selective α4β7 integrin inhibitor designed for the potential treatment of patients with IBD. The Phase 1 clinical program for NSHO-101 evaluated 184 healthy subjects to assess safety, tolerability, food effects, pharmacokinetics (PK) and pharmacodynamics (PD) of single and multiple ascending doses. NSHO-101 demonstrated target engagement in this Phase 1 program. There were no appreciable increases in peripheral lymphocytes, suggesting its activity is gut-restricted. NSHO-101 was generally safe and well tolerated.
We plan to initiate Phase 2 clinical development of NSHO-101 as a potential treatment for ulcerative colitis (UC) in the first half of 2025.
Leadership
Our leadership team will include a seasoned team of executives with decades of experience in the discovery, development, and management of biotechnology companies in the inflammatory disease and IBD space.
Neena Bitritto-Garg, CFA
Founder, President & Chief Executive Officer, and Executive Chair
Danielle Fry, JD
Chief Operating Officer and Chief Legal Officer
Matthew McKevitt, PhD
Chief Development Officer
Andy Whitney, PhD
Chief Scientific Officer
Bittoo Kanwar, MD
Chief Medical Officer
Emily Weng, ScD
Chief Data Science Officer
Chrissa Ann Rizk
Senior Vice President, Head of Clinical Operations
Neena Bitritto-Garg, CFA
Founder, President & Chief Executive Officer, and Executive Chair
Neena Bitritto-Garg is the Founder, President & Chief Executive Officer, and Executive Chair of Ensho.
Ensho was borne out of Neena’s experience working at Eisai, Co. Ltd., the parent company of EA Pharma Co., Ltd. (from which Ensho’s pipeline was in-licensed), on business development and in management of the company’s key alliance with Biogen on Leqembi (lecanemab), which is currently being launched in multiple countries for the treatment of early Alzheimer’s disease.
Neena also brings nearly a decade’s worth of experience as an equity research analyst covering the biotechnology sector, including the neuroscience and immunology spaces. Immediately prior to founding Ensho, she led biotech and biopharma equity research strategy at multiple global banks, including at Citigroup Inc., where she was the Head of U.S. Biotechnology Equity Research.
Neena is a graduate of the Roy and Diana Vagelos Program in Life Sciences and Management (LSM) at the University of Pennsylvania and holds dual degrees in Biochemistry (B.A.) from the College of Arts and Sciences and Economics (B.S.) with a concentration in finance from Wharton.
Danielle Fry, JD
Chief Operating Officer and Chief Legal Officer
Danielle is the Chief Operating Officer and Chief Legal Officer at Ensho, where she brings extensive experience in business development, alliance management, and clinical development. Before joining Ensho, Danielle played a critical role at Eisai Inc. where she supported business development efforts and managed key strategic alliances, including collaborations in clinical development.
Prior to her time at Eisai, Danielle was part of the Reed Smith Life Sciences Transactions group, where she worked on mergers and acquisitions (M&A), licensing agreements, and other high-impact transactions within the life sciences sector.
Danielle began her career as a scientist at Schering-Plough, where she conducted research in HIV and Hepatitis C, contributing to the development of key therapeutic programs. Her hands-on experience in scientific research provided her with a deep understanding of the complexities of drug development and clinical trials.
Danielle holds a B.S. in biology from The College of New Jersey and following her time at Schering-Plough, obtained a law degree (J.D.) at Seton Hall Law School, where she honed her expertise in life sciences transactions and regulatory affairs. This unique combination of scientific and legal knowledge has been pivotal in her roles across the pharmaceutical and biotech industries.
Matthew McKevitt, PhD
Chief Development Officer
Matthew is the Chief Development Officer of Ensho. Matthew brings to Ensho 16 years of extensive experience in clinical research and development, including execution of preclinical and Phase 1-3 studies in IBD as well as Phase 1-4 studies in respiratory therapeutics. He most recently served for the past four years as Senior Director at Gilead Sciences as a Global Development Lead, transitioning IBD assets from preclinical to Phase 1 and 2 clinical development. Prior to that, he spent 12 years at Gilead as a Clinical Research Scientist, where he acted as a clinical research lead, study director or translational lead involving Crohn’s Disease, UC, and respiratory disease studies. Prior to Gilead, Matthew spent 10 years in academic research in microbiology, pathogenesis, biochemistry, molecular biology, vaccine discovery, high-throughput genomic and proteomic experimentation, in vitro/in vivo experimentation as well as pre-clinical experimentation in BSL2 and A/BSL3 lab settings.
Matthew holds a Ph.D. in Biochemistry from Baylor College of Medicine, a B.S. in Biochemistry from Seattle University, and a B.S. in Philosophy from Willamette University.
Andy Whitney, PhD
Chief Scientific Officer
Andy Whitney is the Chief Scientific Officer of Ensho. Andy brings extensive experience in autoimmune/inflammatory, oncology and rare diseases drug discovery and development. He has a proven track record building and leading dynamic and successful research teams in both small and large companies. Andy has extensive project leadership experience, with multiple programs advancing from target selection through clinical development and has contributed to the successful development of two U.S. FDA-approved therapeutics.
Andy’s 25 years of experience includes leading the preclinical drug development as Senior Vice President, Head of Preclinical Development and Translational Science at Applied Molecular Transport, and prior to that, leading preclinical biology efforts for multiple rare disease projects as Vice President, Biology at BridgeBio. Previously, Andy was a Director in Gilead’s inflammation and oncology research group, where he held site leadership roles and led research efforts for multiple small molecule programs in inflammation and oncology. He was previously one of the earliest staff at CGI Pharmaceuticals, Inc., where he led biology, bioinformatics and project teams until its acquisition by Gilead.
Andy holds an A.B. from Harvard College and a Ph.D. in Cell Biology from Yale, and was an National Science Foundation postdoctoral fellow at the University of Geneva, Switzerland.
Bittoo Kanwar, MD
Chief Medical Officer
Bittoo Kanwar is the Chief Medical Officer of Ensho. Bittoo brings to Ensho broad industry experience and strong relationships in the immunology and inflammation space. Most recently, Bittoo was the Chief Medical Officer of Telavant Holdings, Inc., a Roivant company acquired by Roche in 2023, where he oversaw the Phase 3 clinical development of RVT-3101, an anti-TL1A antibody, as a potential treatment for ulcerative colitis (UC) prior to the acquisition by Roche.
Prior to Telavant, Bittoo was Chief Medical Officer at Applied Molecular Transport, Inc., where he led the company’s IBD-focused clinical development programs. Earlier in his career Bittoo served as Vice President, Head of Development at Protagonist Therapeutics, Inc., leading clinical programs in UC and Crohn’s Disease. He began his industry career at Gilead Sciences, Inc., where he served as the Clinical Domain Lead for Inflammatory Bowel Disease and supported programs in liver fibrosis/NASH, Hepatitis B and C. Prior to his career in industry, Bittoo was an Associate Professor at the University of California, San Francisco (UCSF), focused on researching the role of the gut adaptive immune response in both HIV infection and inflammatory bowel disease (IBD).
Bittoo holds a B.S. from the University of Minnesota and an M.D. from the University of Iowa. He completed his residency at Rush University Hospitals in Chicago and a fellowship in pediatric gastroenterology at UCSF.
Emily Weng, ScD
Chief Data Science Officer
Emily is the Chief Data Science Officer of Ensho. Emily brings to Ensho two decades of expertise in applying statistical and design methods throughout the diverse aspects of the research and development programs across broad therapeutics pipelines in the biopharmaceutical industry, including immunology and inflammatory disease.
Emily most recently served as Vice President, Head of Biometrics and Data Science at Telavant, a Roivant company acquired by Roche in 2023, where she provided strategic leadership and operational oversight in her functions to support the company’s product development program of RVT-3101, an anti-TL1A antibody, as a potential treatment for ulcerative colitis (UC). Prior of Televant, Emily spearheaded the biometrics program at Applied Molecular Transport where she built and led biostatistics, clinical data management, and programming functions to support multiple IBD development programs. Prior to AMT, Emily was Senior Director, Biometrics, and Statistical Head of Respiratory TA at Theravance Biopharma, Inc., and Director, Biostatistics at Allergan Plc, acquired by AbbVie in 2020. Over the course of her career, she and her team accomplished multiple regulatory submissions and approvals of new pharmaceutical products.
Emily holds an Sc.D. in Cancer Biology with a minor in Biostatistics and an M.S. in Epidemiology from Harvard T.H. Chan School of Public Health and an Executive M.B.A. from the UCLA Anderson School of Management.
Chrissa Ann Rizk
Senior Vice President, Head of Clinical Operations
Chrissa is the Senior Vice President and Head of Clinical Operations at Ensho. Chrissa brings to Ensho decades of experience in the pharmaceutical and biotechnology industries, leading and executing successful clinical operations in IBD clinical development as well as a multitude of other therapeutic areas.
Her experience includes global execution, strategy and management of the UC program at Televant, a Roivant company acquired by Roche in 2023, and several IBD programs at Genentech. While at Genentech, she also oversaw a broad spectrum of clinical programs within cardiovascular, neuroscience, infectious disease, and immunology. Chrissa was also a key member of the clinical operations team at Bristol-Myers Squibb, providing strategic leadership and support of ongoing global Phase 1 – 3 studies.
Chrissa holds a B.S in Comprehensive Science from Villanova University.
Recent News
June 27, 2024
Ensho Therapeutics Launches with Phase 2-Ready Oral α4β7 Inhibitor for Inflammatory Bowel Disease
